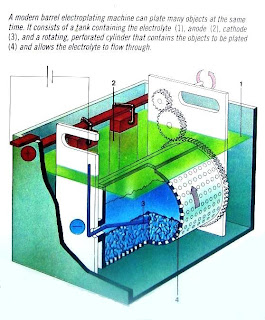
 |
| Automatic Bath |
BASIC PROCESS ELECTROPLATING
Electroplating is the deposition of a metal onto a metallic surface from a solution by Electrolysis decoration and protection of the metal. Metals commonly use to plate surface are silver, chromium, cadmium, zinc, gold and copper. Each metal as coating have specific solution that can work effectively. In the below example copper have chemical symbol of Cu, and after dilute in the solution can become ion and give the symbol Cu++. Because Cu have ion in the solution. In the case of copper platting for example electroplating takes place by means of the reaction of
process. It is used for purpose of
Cu++ + 2e ====> Cu. Cu++
in this equation represent an ion that is carried to the metal surface to be plated, known as the CATHODE, from the source of the metal being plated, known as ANODE. The ion forced to the cathode by an external source such as a battery. The electrolytic solution is a salt of the metal being plated; in the case of copper plating. It is copper sulfate, CuSO4.5 H2O.
The appearance, adhesion, porosity, and protection value of electroplated coatings depends on several things, including the type of base metal be plated, the preparation of the metal to be plating, and the electrodepositing process itself.
Base Metal Use for Electroplating
It is not possible to apply high quality coatings to metals of poor quality. The composition of the base metal is usually known and controlled. The condition of the surface, including the degree and nature of the polishing processes, may also have a direct bearing on the characteristic and porosity of the coating.
Preparation for Metal Plating
It is necessary to have the base metal surface chemically clean in order to secure good adhesion of deposits. The preparation usually involve cleaning that is, removal of grease and foreign particles and picking in the case of steel, or remove oxides or other compounds, and is some instances, to etch the surface. Because of a thin, natural oxide film, aluminum alloys do not usually respond to the cleaning and surface preparation treatments employed for other metals; special techniques must be used for such alloys.
Electrodeposition of Electroplating
 |
| Metal Ion in solution |
Finally, the anodes should ordinarily be of such composition and structure as to maintain the metal concentration and the pH. Where insoluble anodes are used, the metal content is maintained by replenishment.
Most commercial plating with potentials of conducted with motor generator sets, usually with potentials of from 6 to 12 volts, and with current outputs, depending on the area of work to plated and the current density.